I saw a news on TIME website a couple days ago that a vaccine against the dengue virus (DENV) is approved by the World Health Organization (WHO). This is something big because DENV infection could affect nearly half of the world’s population. Thanks to the climate change, we might see a bump in that number because the mosquito carrying DENV now can access regions previously uncharted by that vector.
Here I want to share something about the design of the DENV vaccine. Commercially known as Dengvaxia, its scientific name is actually CYD-TDV. Let’s understand its scientific name because it is much more meaningful to me.
it is a chimera
The first half of the name, CYD, stands for chimeric yellow fever virus-DENV. Why? Because the vaccine is constructed by using yellow fever (YFV) viral backbone, strain 17D (YF17D). Why we use YFV as the viral backbone? There are two answers for this. First, YFV and DENV are of the same family: Flaviviridae. Thus, their behavior could be similar in certain ways. Second, it is because the immunogenicity and safety profile of the YF17D is well documented and scientists have a good grip on its biology. It is generally safe being attenuated, hence it is desirable to apply an already existing knowledge without reinventing the wheel.
Since we have the genetic code for constructing a complete YF17D viral particle. What do we do next? Let’s do some genetic manipulation.
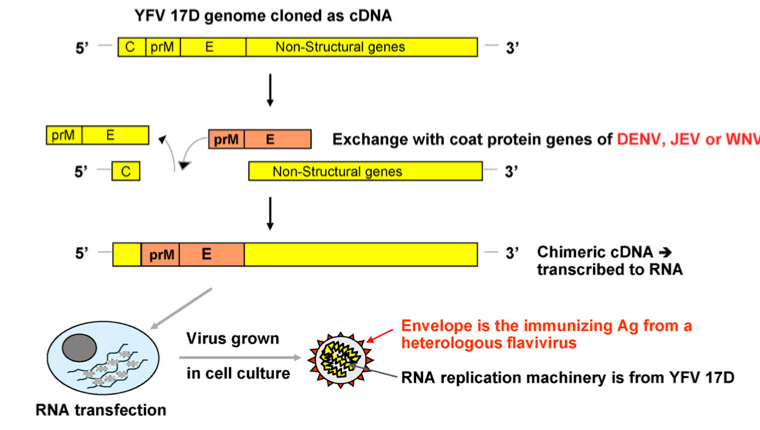
Generally speaking, our antibody tends to recognize the structural proteins that give a virus its shape. We call these proteins as capsid and/or coat proteins. Our antibody does not really care what’s inside, with an exception to some cases. To construct a vaccine for flaviviruses, we just need to swap two genes on the YF17D backbone: prM and E genes. In our case here with Dengvaxia, we need to replace prM and E genes from the original YF17D to the DENV version of the prM and E genes. Now our YF17D is wearing DENV’s clothes.
Neat, right?
it is a multi-valent vaccine
There are 4 distinct serotypes of DENV currently circulating worldwide. This is where the story gets complicated.
Imagine this hypothetical scenario. You got infected with the DENV serotype 1, you fought it well and weeks later you were fully cured of the infection. Within the next few months since the first infection, your body produces an adequate protection against DENV of all four serotypes. Unfortunately, a full protection won’t last long.
Months later the protection against serotype 2, 3, and 4 wanes while protection against serotype 1 would still be potent. If you contracted another infection with DENV-1, you won’t be in a deep trouble. But there would be a different story if you got infected with serotypes other than DENV-1. The DENV-1 antibody can still recognize DENV-2,3,4 but it is not protective enough to mount a full-scale protection. Your body is tricked. Your body thinks it is doing well because it assumes that DENV-1 antibody is fighting well to an extent you body over-produces DENV-1 antibody but DENV-2,3,4 isn’t neutralized.
Then what would happen?
The fever gets worse, internal bleeding happens, and without a proper medical treatment you could die. This situation is called antibody-dependent enhancement (ADE), because not only your body fails to respond to the invasion but it helps the virus to ravage your body. Hence, the strategy here is to design a vaccine that confers protection against all four serotypes. Since there are four distinct DENV serotypes, we need a tetravalent vaccine. This is where the second half of the name comes from: TDV, which stands for tetravalent dengue vaccine.
Would a single shot be enough or we need some sort of booster? Apparently, we do need booster.
seroconversion
When constructing a vaccine, when it comes to conferring protection we must understand the capability of a vaccine to induce seroconversion. For the sake of brevity, the definition of seroconversion I am using here is that the capability of an antibody to neutralize its antigen. If seroconversion took place or is currently taking place, it means that the antibody is present.
But now, the question is how much the antibody is currently present and how protective the antibody is?
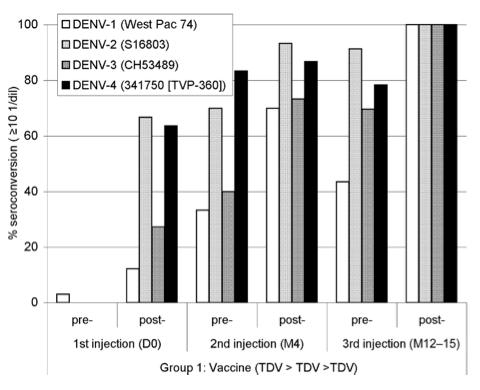
A vaccine is not useful if a single dose will only be capable of producing a small amount of circulating antibody. From the figure above, 3 doses of Dengvaxia must be administered over a 1-year period to introduce a satisfactory amount of antibody. The method for quantifying the amount of antibody here is done by using the PRNT assay, which stands for plaque reduction neutralization test, developed in 1967 by Russell and Nisalak.
conclusion
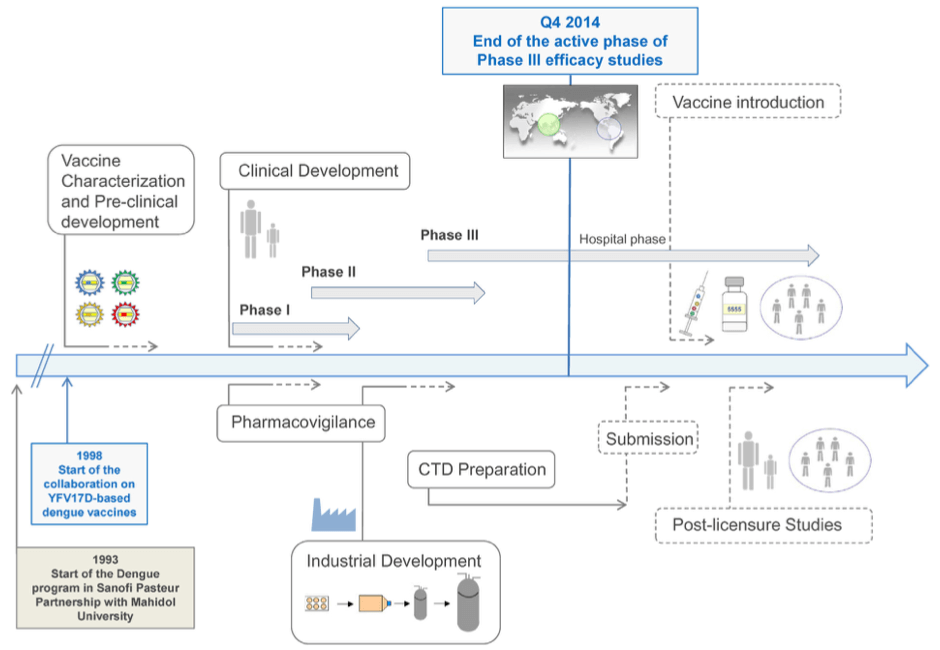
It took Sanofi-Pasteur about 20 years to design and improve the Dengvaxia. Let’s appreciate this vaccine to make sure DENV will be a relic of our past.
Source:
- A Novel Tetravalent Dengue Vaccine Is Well Tolerated and Immunogenic against All 4 Serotypes in Flavivirus-Naive Adults, DOI:10.1086/649916
- Development of the Sanofi Pasteur tetravalent dengue vaccine, DOI:10.1016/j.vaccine.2015.09.108
- Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses, DOI:10.1016/j.vaccine.2009.09.098
- Dengue vaccine: hypotheses to understand CYD-TDV-induced protection, DOI:10.1038/nrmicro.2015.2
- Dengue vaccines: recent developments, ongoing challenges and current candidates, DOI:10.1586/14760584.2013.815412
- Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease, DOI:10.1056/NEJMoa1506223
- Live virus vaccines based on a yellow fever vaccine backbone, DOI:10.1016/j.vaccine.2014.10.004
- Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses, DOI:10.1016/j.vaccine.2009.09.098
- Recent advances in dengue pathogenesis and clinical management, DOI:10.1016/j.vaccine.2015.09.103